DPP® SARS-CoV-2
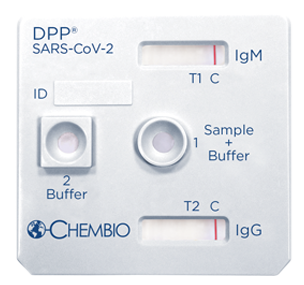
An ANVISA Approved rapid test for the detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in capillary “fingerstick” whole blood, venous whole blood, serum and plasma samples. This post vaccination test detects antibodies to the Receptor Binding Domain (RBD).
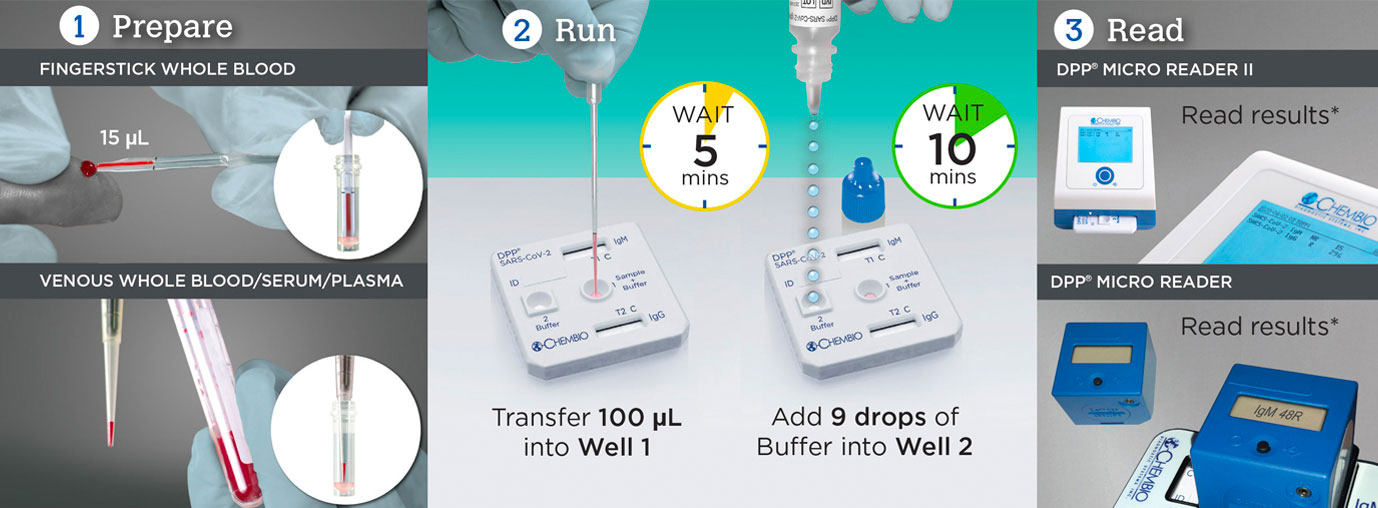
est for SARS-CoV-2 in 3 Easy Steps with DPP® SARS-CoV-2 IgM/IgG
Product Information
| Information Type | Product Detail | |
| Method | Dual Path Platform | |
| Sample | Fingerstick blood, venous whole blood, serum, plasma | |
| Time to Results | 15 Minutes | |
| Storage Conditions | 2°C to 30°C (36°F to 86°F) | |


